Over the past five years, AccessWorld has periodically published the results of projects at AFB TECH that have evaluated the accessibility and usability of diabetes self-care devices. You can read about blood glucose monitors in the September 2002 issue, insulin pumps in the March 2004 issue, and blood pressure monitors in the September 2004 issue. This article examines insulin pens, another device that can be used to manage diabetes. We have gathered and evaluated all eight pens that are currently on the U.S. market.
What Is an Insulin Pen?
For people with diabetes who are required to use insulin, an insulin pen provides a delivery method that is easier, less painful, and more discreet than drawing doses from a vial using a needle and syringe. Insulin pens are small, lightweight plastic handheld devices with prefilled insulin cartridges inside, and they use small microfine needles that have been shown to cause significantly less pain than conventional syringe needles. They all have dials to set the amount of insulin for each dose. Three of them have small electronic displays that show the size of the dose, and five of them show the dosage on the mechanical dial itself, similar to the volume knob on some radios. Five of the units that we tested are reusable, with replacement cartridges available when the insulin runs out, and the other three are disposable and are to be thrown away when the insulin is gone. Each pen can be used only with specific types of insulin (see the Product Features section at the end of this article to learn the type of insulin that is available for each pen). All the pens have windows for viewing the remaining insulin.
The eight insulin pens that we evaluated for this article represent the entire U.S. market: six pens manufactured by Novo Nordisk, one pen manufactured by Eli Lilly, and one pen manufactured by Sanofi-Aventis.
Why Evaluate Insulin Pens?
We at the AFB TECH product evaluation lab have been evaluating diabetes self-care devices over the years because of the close relationship between diabetes and blindness. Nearly one-third of the 21 million Americans with diabetes have some degree of vision loss, and diabetes is the leading cause of blindness among working-age adults. The accessibility and usability of insulin pens is important because, according to the U.S. Department of Health and Human Services, 28.7% of Americans with diabetes require insulin injections. Not all persons with diabetes can or want to use an insulin pump to deliver their insulin, and an insulin pen is an attractive alternative to using a regular syringe.
A 2003 study on microneedles conducted at Georgia Tech and published in Georgia Tech Research News found that the microneedles that are used with insulin pens are considerably less painful to inject than are conventional hypodermic needles because the microneedles are too small to stimulate nerve endings. In addition, a 1998 study in the United Kingdom ("Accuracy and Reproducibility of Low Dose Insulin Administration Using Pen-Injectors and Syringes") found that insulin pens deliver more accurate doses of insulin than do syringes. In a 2002 study conducted at the University of Pittsburgh ("When Oral Agents Fail: Practical Barriers to Starting Insulin"), 87% of the participants described the insulin pen as easier to use than a syringe, and 85% found the pen to be more discreet than a syringe. Greater accuracy of insulin delivery is essential for successfully managing diabetes, and a person is much more likely to do what is necessary to manage diabetes properly if his or her device is less painful, easier to use, and more discreet. Certainly, in a public place, such as a restaurant, a person would be more comfortable using a device that looks like a regular writing pen than using a syringe. The results of our evaluation show how easily a person who is blind or has low vision can access these advantages of insulin pens.
How We Evaluated the Pens
We listed the tasks involved in properly using an insulin pen and evaluated each pen to find out how easily a person who is blind or has low vision could perform each task. We looked at each task and determined if it could be performed by touch alone or if vision would be required. We also investigated how easily a person with low vision could read the display information on the pens that have electronic displays and could perform the other tasks that require vision. In addition, we evaluated the accessibility of the print and electronic manuals from the perspectives of both people who are blind and those with low vision. Finally, since diabetes care can be a complex issue requiring medical oversight and acquiring insulin pens requires a prescription, we sought assistance and guidance from the Marshall University Diabetes Center in Huntington, West Virginia.
The following are brief descriptions of each pen.
The Novolog FlexPen, from Novo Nordisk, is a disposable prefilled pen weighing 0.9 ounces with a full insulin cartridge and measuring 6.2 inches long with a 0.6 inch diameter. It is designed to look like a writing pen, with a normal pen cap. Removing the cap reveals the point where the needle is to be connected, and the dosage dial is at the bottom end of the pen.
Caption: The FlexPen is designed to look like a writing pen until the cap is removed.
The Humalog, from Eli Lilly, is a disposable prefilled pen weighing 1.1 ounces with a full insulin cartridge and measuring 6.3 inches long with a 0.9 inch diameter. It is also designed to look like a pen.
Caption: The Humalog is also designed to look like a pen.
The Novolin InnoLet, from Novo Nordisk, is a disposable prefilled pen weighing 1.6 ounces with a full insulin cartridge and measuring 4.5 by 2.2 by 1.1 inches. It is similar in appearance to an egg timer, with a large dosage dial on the front and the needle at the bottom.
Caption: The InnoLet looks more like an egg timer.
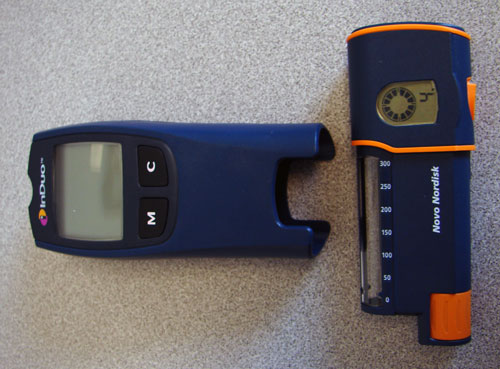
The InDuo, from Novo Nordisk, is a reusable pen weighing 4.7 ounces with a full insulin cartridge and measuring 4.9 by 2.1 by 1.2 inches. It is unique in that it includes a blood glucose monitor. The pen is a small rectangular device with the dosage dial and needle on the same end. It also has an LCD (liquid crystal display) screen on the opposite end. The cap of the pen is built into the blood glucose monitor, which is actually a modification of the One Touch Ultra that was reviewed in the July 2002 issue of AccessWorld. It is a top-rated monitor using modern testing technology, but it does not have speech output or large display information to accommodate users who are blind or have low vision.
Caption: The InDuo is unique in that it includes a blood glucose monitor.
The Innovo, from Novo Nordisk, is a reusable pen weighing 2.7 ounces with a full cartridge and measuring 4.7 by 1.6 by 0.8 inches. It is a small rectangular device with the dosage dial and needle on the same end and an LCD screen on the opposite end. It is actually the same pen as the InDuo without the blood glucose monitor.
Caption: The Innovo is the same pen as the InDuo, without the blood glucose monitor.
The Novopen 3, from Novo Nordisk, is a reusable pen weighing 2.3 ounces when full and measuring 6.4 inches long by 0.6 inches in diameter. It is designed to look like a pen, with the dosage dial at the bottom and the needle at the top.
Caption: The Novopen 3 is another insulin pen that is designed to look like a writing pen.
The Novopen Junior, from Novo Nordisk, is a reusable pen weighing 2.3 ounces when full and measuring 6.4 inches long with a 0.6 inch diameter. It is actually the same pen as the Novopen 3, but it has been modified for children, having the capability of delivering doses in half-dose increments.
Caption: The Novopen Junior is the same pen as the Novopen 3, but has been modified for children to deliver half-doses.
The OptiClik, from Sanofi-Aventis, is a reusable pen that is distributed free of charge by health care providers to their patients with diabetes. Weighing 2.1 ounces when full, it measures 6.8 inches long by 0.8 inches in diameter. It is designed to look like a pen, with the dosage dial at the bottom and the needle at the top. Near the dial is an LCD screen that displays the number of units that are dialed.
Caption: The OptiClik is designed to look like a pen when the cap is on and has an LCD screen near the dial.
Results
In presenting our results, we have separated the disposable pens from the reusable pens. First we examine all the tasks involved in using the pens and discuss how accessible each pen is in relation to each task. After the task analysis, we discuss the accessibility of the display screens and other vision-related tasks, followed by the results of the evaluation of the user manuals.
Accessibility of the Disposable Pens
Because there is no need to change insulin cartridges with the disposable pens, these pens require fewer tasks and are easier to use. Each of the three reusable pens requires you to perform only the following eight tasks:
Task 1: Remove the cap from the body of the pen
We found no particular accessibility barriers with this simple first task.
Task 2: Safety precautions
Before injecting, you must take two safety precautions. First, you must gently move the pen back and forth in your hand to mix the insulin properly. We found no barriers to performing this task, but you must then visually inspect the gauge on the side of the cartridge to determine if there is enough insulin remaining for your desired dose. This task, of course, cannot be performed by people without sufficient vision, and the manuals all state that you should get rid of the pen if you cannot tell if there is enough insulin for your required dose. One possible work-around for this problem would be to keep track of exactly how much insulin you have used, including the amount used for the priming process described under Task 4. Each pen comes with 300 units of insulin, and you may be able to keep a running total, subtracting from the 300-unit starting point.
Task 3: Remove the protective seal from the needle, connect the needle to the pen, and remove the caps covering the needle.
This task is accessible, but you must take care not to stick yourself or otherwise contaminate the needle.
Task 4: Prime the pen
This task is done by setting the pen to deliver a dose of two units and then inspecting the end of the needle to determine if insulin was actually expelled. Setting the two-unit dose and delivering the insulin is not inaccessible, but it is nearly impossible to determine nonvisually whether any insulin was delivered. You cannot safely touch the end of the needle to feel for the liquid insulin, and you would risk contaminating the needle by touching it. You could set it to deliver several units and try to aim it to drop on your fingertips, but you would be wasting a lot of expensive insulin.
Task 5: Set your required dose
This process is the same as for priming the pen. On the FlexPen and Humalog, you pull out the dial on the bottom of the pen. You then twist the dial clockwise, listening for and feeling the clicks and counting as you twist. Each click represents one unit of insulin. With the InnoLet, you just rotate the egg-timer type of dial to the right and listen and feel for the clicks, counting as you go. This task is accessible, but it requires concentration to count the clicks accurately as you set your dose. If you are interrupted or otherwise lose count, you can simply dial the pen back until it stops, and you will be at zero and ready to start over. The FlexPen provided the best auditory and tactile feedback, with the Humalog slightly behind and the InnoLet in third. Because its egg-timer type of dial has raised marks to represent every five units along the dial, the InnoLet's egg-timer dial is potentially the most accessible. However, the tactile and auditory feedback were not what they could be, and the raised markings are too far away from the pointed end of the dial for our testers to be confident that, for example, they had correctly dialed in 10 units, not 9 or 11 units. In addition, it is not easy to determine tactilely the difference between the right and wrong ends of the dial pointer.
Task 6: Deliver the insulin
Once the insulin dose has been set, use the injection technique shown by your health care provider to deliver the insulin. You insert the needle into your skin and press in the push button above the dial completely. Let the needle remain in the skin for several seconds to ensure that all the insulin has been delivered. This task presents no accessibility barriers, as long as you have learned the proper technique from your physician or certified diabetes educator.
Task 7: Remove the needle
Pull out the needle and then unscrew and properly dispose of the pen needle. This step also presents no accessibility barriers, but you should always be cautious when needles are involved. Also, do not reuse needles, since a new needle is required for every injection.
Task 8: Put the pen cap back on
Obviously, this task is accessible.
To sum up the accessibility results for the disposable insulin pens, we found three main barriers that can affect the ability of a person who is blind or has low vision to use these pens independently. First, there is the need to inspect the gauge on the insulin cartridge visually to make sure that enough insulin is remaining. Second, there is the difficulty, when priming the pen, of determining whether any insulin was ejected from the needle. Finally, users may have concerns about accurately setting a dose when they rely only on tactile and click feedback, especially in a noisy situation or when setting a large dose. Sighted users can rely on the visual feedback provided by the dials in addition to the clicks.
Accessibility of Reusable Pens
When evaluating the reusable pens, we found the same three main barriers that we indicated with the disposable pens. However, we found some additional difficulties that affect the accessibility and usability of the reusable pens, so that these pens are slightly more difficult to use. Other than replacing the cartridges, the tasks involved are the same as those described for disposable pens, so we will discuss only the cartridge-replacement tasks and those that present additional accessibility barriers. The InDuo and Innovo function identically, as do the Novopen 3 and Novopen Junior.
Replacing the insulin cartridge
The main problem with changing the insulin cartridge is knowing when the insulin is low and it is time for a replacement because just as with the disposable pens, this information must be determined visually. Then, although they definitely take some training and practice with your health care provider, the steps involved in actually changing the insulin cartridge are accessible on all the reusable pens. All the important parts of the pens are tactilely identifiable, and all the tasks can be performed nonvisually as long as you have learned the process. However, several tasks are involved, and they do pose some difficulty:
- Unscrew and remove the protective covering over the old cartridge.
- [For Novopen 3 and Junior only] Reset the plunger by separating the individual parts of the pen.
- Place the cartridge upright in the proper place and replace the protective covering.
We found changing the cartridge to be the most difficult with the Novopens because they require one extra step.
Additional accessibility barriers
The additional problems we found with the reusable pens mainly involve setting the dosage, particularly setting the dial back to zero if you miscount or get distracted. As we noted earlier, the disposable pens have an absolute zero point where the dial stops, so you cannot go any further and know you are back at zero. However the InDuo/Innovo pens do not stop at zero, and the dial can keep turning past zero. Thus, you have to rely on viewing the display screen to know that you have successfully returned the dial to zero and are ready to start over. With the OptiClik, you also have to rely on viewing the display when setting it back to zero. Although it does have a hard stopping point where you cannot turn the dial back anymore, it did not consistently stop at the zero point. During our testing, the OptiClik would often stop at the one- or two-unit points, so users have to check the display screen visually to confirm that it returned to zero. If it does not return to zero, you have to press the button to deliver the insulin and start over, which wastes insulin. The problem with the Novo Pens is that if you try to turn the dial back toward zero, the pens actually begin to deliver the insulin. To avoid this problem, you have to pull the pen apart, separating the dial from the part with the cartridge, and then dial it back to zero.
Accessibility of Documentation
Because of the serious consequences of the improper use of insulin pens, it is imperative that the user manuals for these pens be accessible. However, we found a bit of a mixed bag when evaluating them. All the manuals were available in electronic format, with six of the manuals in PDF format and the InnoLet and OptiClik manuals in HTML format. The text in the PDF manuals was accessible using screen-reader software, and the manuals included a lot of information on the safety concerns of diabetes management. However, the diagrams of the pens and their various parts were not accessible, since they relied on graphical representations. There were also some instances of unlabeled graphics when step-by-step instructions were provided. Similar problems were seen in the HTML manuals, and the OptiClik page had some unlabeled graphical links. We found no braille manuals for any of the pens.
The good news regarding access to the manuals by people with low vision is that the electronic documentation is fully compatible with screen-magnification software. The bad news is that the print in all the instruction manuals is fairly small. In fact, the largest print manual is in 12-point font, which is too small for many people with low vision to read. In general, these manuals were more accessible than are the majority of device manuals that we have seen over the years at AFB TECH. However, when it comes to medical devices, the information in the manuals should be 100% accessible because of the seriousness of the safety concerns.
Low Vision Accessibility
Our low vision accessibility experts assessed both the visual aspects of the insulin pens and the print and electronic documentation. With regard to the insulin pens themselves, some are easier to use by people who are visually impaired than are others. Of the insulin pens that we evaluated, the two that are the most difficult to use are the Humalog Pen and the OptiClik because their displays are the hardest to see. The Humalog Pen has a small, round magnifying bubble over the number indicating the number of insulin units you have dialed up. The bubble casts shadows and glare over the already-small numbers, making them difficult to see. Similarly, the OptiClik has a small LCD display that is covered by a clear covering that is curved to match the shape of the pen. The curve of the LCD covering casts a glare over the already-small digital numbers, making them difficult to be read by many people with low vision. The OptiClik also gives its reading in a two-digit number; for example, five insulin units would be designated by a reading of 05. This format can be distracting to a person with low vision; removing the 0 from the readout on numbers less than 10 would be an improvement.
Of the insulin pens that we evaluated, the ones with the most visible displays were the InnoLet, the InDuo, and the Innovo. The InnoLet has a large, round, egg timer-style display, and you turn the dial to the number of insulin units that you want to dial up. The audible clicks as the dial passes each number also give the user cues that confirm that the number has been reached. The InDuo, the combination blood glucose monitor and insulin pen, has a small LCD display, although it is more readable than others with small dials that we evaluated. The odd thing about the InDuo is that the blood glucose monitor has a large LCD display and large numbers. If the manufacturer thought it was important to make the blood glucose monitor with an easy-to-read LCD display, why wouldn't it make the insulin pen portion with a large, easy-to-read LCD display as well? The Innovo is identical to the insulin-pen portion of the InDuo and has the same size LCD display.
The Bottom Line
Although we found the insulin pens to be relatively easy devices to use, especially compared to using an insulin pump, they all still have serious accessibility barriers that get in the way of a person who is blind or has low vision using them independently as part of his or her diabetes care regimen. Inspecting the remaining insulin level; priming the pen; and, to a lesser extent, setting the dose all rely on a person using his or her vision. The reusable pens also have the added problems with resetting the dosage dial back to zero and replacing the cartridge. Thus, people who are blind or have low vision have to rely on sighted assistance to use an insulin pen safely, which is not ideal diabetes self-care. If you have to rely on others, you are most likely not going to do the best job of managing your diabetes.
That being said, many of the tasks involved in using insulin pens are accessible, and we have compiled a list of the pens in the order of how usable they are. Because of the fewer tasks that are involved with disposable pens and the additional barriers found in reusable pens, the disposable pens rate the highest. However, readers should be aware that reusable pens are more cost-effective in the long run. Although reusable pens cost more in the beginning, their insulin replacement cartridges are less expensive than having to buy a new disposable pen every time.
Novolog FlexPen gets our top rating because of the solid tactile and auditory feedback it provides when setting a dose.
Humalog is a close second because its tactile and audio feedback are nearly as good as those of the FlexPen.
Although the InnoLet's egg-timer style of dial is potentially the most accessible style of dosage dial, it comes in third because it has less auditory and tactile feedback. A new design of the dial with a pointier end that reached all the way to the raised tactile markings along the face would greatly improve this pen.
4 and 5. The InDuo/Innovo pens are next because although they have additional usability barriers like the other reusable pens, they provide good audio and tactile feedback.
6 and 7. The Novopens are near the bottom of the list because they have less audio and tactile feedback.
- The OptiClik is at the bottom of the list because the user has to rely too much on the display screen for tasks such as setting the dose and reading the battery level.
Safety and Insulin Pens
We have done this research on the accessibility and usability of diabetes self-care devices over the years both to show manufacturers how they can improve their devices and to inform our AccessWorld readers. We hope we have made you more aware of the issues involved in the use of insulin pens, but we also want to convey the seriousness of properly using an insulin pen. These pens are dangerous if they are used improperly, and we have seen numerous warnings against using them alone if you are blind or have low vision. In fact, the manuals for five of the pens specifically say that they are not designed to be used by people who are blind or have low vision.
We find this situation ironic, since one-third of Americans with diabetes also have some degree of visual impairment. Since these pens are safer and so much easier to use than the old vial-and-syringe method, we hope that manufacturers of devices can design new pens in ways that make them more usable by people who are visually impaired. We have read some research that has suggested the possibility of developing electronic pens. If such pens are actually developed, then perhaps the electronics could include speech-output functionality to monitor the levels of insulin cartridges and to speak the dosage information as you set the dial. Short of going to electronic pens, greater accessibility and usability could be achieved if manufacturers would develop the InnoLet egg-timer type of dial but with some slight improvements. These improvements should include making the pointy, or business end, of the dial contrast better tactilely with the other end and reach all the way to the raised markings on the dial and providing better tactile and auditory feedback when the dial is turned to set the dose. All the pens also need to have larger, easier-to-read displays. Whether they are analog dials or LCD displays, the numbers need to be large and free of clear plastic coverings that cause glare. In addition, all LCD-displayed information should contrast well with its background to improve readability.
In the meantime, people with diabetes who are blind must use sighted assistance to use an insulin pen safely. We would be curious to hear from readers about this issue. Perhaps you could send us any techniques you may have developed for delivering your insulin. One such technique was passed on to us by Anise Nash, the certified diabetes educator who advised us on this project. She said that people with diabetes may have to use more than one type of insulin, such as a rapid-acting and a long-acting insulin. Since both types may be prescribed in identical pens, she suggests that you place a rubber band around the pen with the rapid-acting insulin to differentiate it tactilely from the other pen. She also said that the Novo Nordisk pens use different colors and tactile markings on the plunger buttons of their pens to indicate the different insulin types.
Finally, we want to stress that if you have diabetes, you should always consult your health care provider about insulin delivery, and please do not rely on this article alone.
This is just a general overview of the accessibility and usability of insulin pens and should not replace the information provided by your certified diabetes educator or physician. This article should make you more aware of your options when consulting your health care provider, and you should proceed only under the direction of your physician or certified diabetes educator.
Note: Just prior to publication of this article, Novo Nordisk stopped producing the InDuo. We reported on it in this article, however, because it is still being sold by some distributors and is still being used by many people. The supplies for it are also still being sold.
View the Product Features as a graphic
View the Product Features as text
View the Product Ratings as a graphic
View the Product Ratings as text
Product Information
Products: InDuo, Innovo, Novolin InnoLet, Novolog FlexPen, Novopen 3, Novopen Junior.
Manufacturer: Novo Nordisk, 100 College Road West, Princeton, NJ 08540; phone: 800-727-6500; web site: <www.novonordisk.com>.
Prices (for pens; prices for replaceable cartridges [RC], when applicable, are in parentheses): InDuo, $99.00 (RC, $17.96); Innovo, $57.95 (RC, $17.96); Novolin InnoLet, $13.60; Novolog FlexPen, $31.96; Novopen 3, $24.99 (RC, $17.96); Novopen Junior, $37.49 (RC, $17.96).
Product: Humalog.
Manufacturer: Eli Lilly, Lilly Corporate Center, Indianapolis, Indiana 46285; phone: 800-545-5979; web site: <www.lillydiabetes.com>.
Price: $30.03.
Product: OptiClik.
Manufacturer: Sanofi-Aventis, 300 Somerset Corporate Building, Bridgewater, NJ 08807-2854; phone: 800-981-2491; web site: <www.Sanofi-Aventis.us>.
Price: No charge for the pen, but the RC costs $31.73.
Acknowledgements: We would like to acknowledge the valuable assistance to this project provided by AFB TECH's Lee Huffman and Marshall University interns Morgan Blubaugh and Michael Price. Special thanks also to Bruce Chertow, M.D., FACE, FACP, of the Joan C Edwards School of Medicine at Marshall University and the Marshall University Diabetes Center, and Anise Nash, RN, BSN, CDE, CPT, of the Marshall University Diabetes Center. This product evaluation was funded by the Teubert Foundation, Huntington, West Virginia.